Source: The Conversation (Au and NZ) – By Flora Hui, Research Fellow, Centre for Eye Research Australia and Honorary Fellow, Department of Surgery (Ophthalmology), The University of Melbourne
The United States Food and Drug Administration has just approved the first-ever clinical trial that uses CRISPR-Cas13 RNA editing. Its aim is to treat an eye disease called wet age-related macular degeneration that causes vision loss in millions of older people worldwide.
This trial marks a new frontier in gene therapy – the process of treating or curing medical conditions by changing a person’s genes.
What makes it special is the fact the therapy targets RNA, instead of DNA. So, what does that mean, and why should we be excited?
What is gene editing and how is it used?
Genes are made up of DNA, or deoxyribonucleic acid. Nearly all cells in your body have the same DNA, the material that makes your body uniquely yours. If anything goes wrong in your DNA, it can result in various diseases.
Thanks to recent advances, we now have the tools to directly change someone’s DNA – this has paved the way for gene editing as a type of gene therapy.
It is done using the CRISPR-Cas9 system, which was created after scientists discovered that bacteria defend against invading viruses by capturing their DNA and destroying it.
This makes gene editing highly useful when designing new treatments for genetic conditions where you need to correct faulty DNA.
Gene editing has already been trialled in people. Earlier this year, a successful clinical trial was done to test the safety of a new gene editing therapy for an inherited eye disease. Gene editing has also been trialled for a heart disorder called transthyretin amyloidosis, as well as blood disorders.
Gene editing causes permanent changes to a person’s genes, effectively rewriting parts of their DNA. But altering DNA comes with its own challenges and risks.
Care must be taken to avoid accidentally causing unintended but permanent changes to DNA elsewhere in the gene, which could lead to unwanted mutations.
Read more:
What is CRISPR gene editing, and how does it work?
What is RNA and how does RNA editing work?
One way to avoid the risks of editing DNA is to target RNA or ribonucleic acid instead.
RNA is also in all our cells, and plays a key role in their functions. One of its jobs is making proteins. If DNA is the set of genetic instructions, RNA is what reads and translates those instructions into making the proteins our cells need.
RNA editing, then, is also a type of gene therapy. Its goal is to change how RNA interprets genetic instructions to control how proteins are made. In most recent advancements, RNA editing uses the CRISPR-Cas13 system, a newer technique that was created specifically to help develop therapies that work with RNA.
DNA editing is permanent, which is needed to treat genetic diseases. RNA editing events, on the other hand, are transient in nature because RNA molecules are constantly being made and degraded in our cells.
RNA editing doesn’t permanently change a person’s DNA, but rather alters the steps that happen after the RNA molecule “reads” the DNA instructions.
This means it can be used to produce more targeted results by, for example, only altering how one specific protein is made. This also makes it a potentially safer option over DNA editing, with fewer unintended effects on other cells.
RNA editing also has an advantage where you can potentially control or reverse the therapy, providing a level of control DNA editing can’t provide.
This is an important factor to prevent over-treatment and makes it a versatile therapy for conditions where faulty DNA isn’t the cause of the disease.
So what is this first RNA editing trial going to do?
Age-related macular degeneration or AMD affects more than 200 million people worldwide and is predicted to grow to 300 million by 2040.
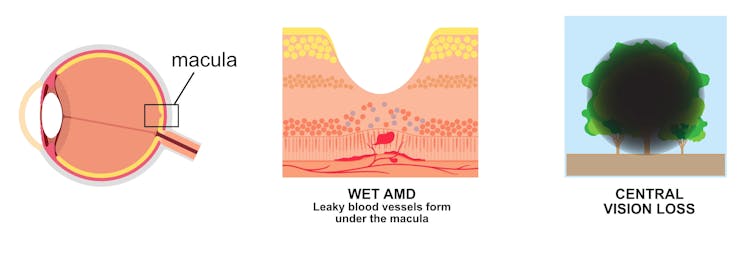
As the name suggests, age plays a role – it almost exclusively affects people older than 55 years. AMD affects the health of the macula, the central part of the retina, which processes what we see. It’s a leading cause of irreversible blindness around the world.
Wet AMD occurs when there is a build-up of fluid and new, leaky blood vessels underneath the macula, causing rapid and severe impact to a person’s central vision.
The Conversation/Shutterstock
Currently, it’s treated with regular drug injections into the eye to control the growth of the leaky blood vessels. The drugs block VEGF, or vascular endothelial growth factor, a molecule that tells our bodies to make new blood vessels.
This is where RNA editing comes in. In the lab, scientists have proven that the delivery of the RNA editing therapy via a safe, engineered virus allowed for an effective reduction of VEGF levels to stop new blood vessel growth in the eye through a one-off injection. For treating wet AMD, it would mean no more monthly needles.
The FDA-approved clinical trial will now assess the safety of RNA editing therapy for wet AMD. It’s also the first-ever clinical stage trial for a CRISPR-Cas13 RNA editing therapy, marking a significant milestone for the field of research.
While it’s early days for the technology, the new trial shows RNA editing therapies have arrived. It will be yet another powerful tool in humanity’s arsenal to develop safe new therapies for various medical conditions.
![]()
The authors do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
– ref. RNA editing is the next frontier in gene therapy – here’s what you need to know – https://theconversation.com/rna-editing-is-the-next-frontier-in-gene-therapy-heres-what-you-need-to-know-243938






