Source: The Conversation (Au and NZ) – By Nial Wheate, Associate Professor of the Sydney Pharmacy School, University of Sydney

Cough medicines containing the active ingredient pholcodine are being withdrawn from sale due to safety concerns highlighted for years both in Australia and internationally.
Yesterday, Australia’s medicines regulator announced the immediate registration cancellation and recall of dozens of these over-the-counter cough medicines and lozenges.
This is because of the risk of a sudden, severe and life-threatening allergic reaction if people are also given specific muscle relaxant drugs while under a general anaesthetic.
That risk of anaphylaxis can remain weeks and months after taking the cough medicine.
Read more:
Still coughing after COVID? Here’s why it happens and what to do about it
What is pholcodine?
Pholcodine (pronounced pho-co-dean) is an opioid-based medicine, which means it’s related to morphine and codeine. It works by binding to various opioid receptors in a part of the brain responsible for triggering the cough reflex.
As such, it is a common ingredient in many over-the-counter medicines used to treat a dry cough. These include cough syrups and lozenges. Every product that contains pholcodine will list it prominently on the bottle or cardboard packaging.
Common brands that contain this ingredient include Benadryl, Bisolvon, Codral, Difflam, Difflam Plus and Duro-Tuss.
Read more:
Sore throats suck. Do throat lozenges help at all?
Why the recall?
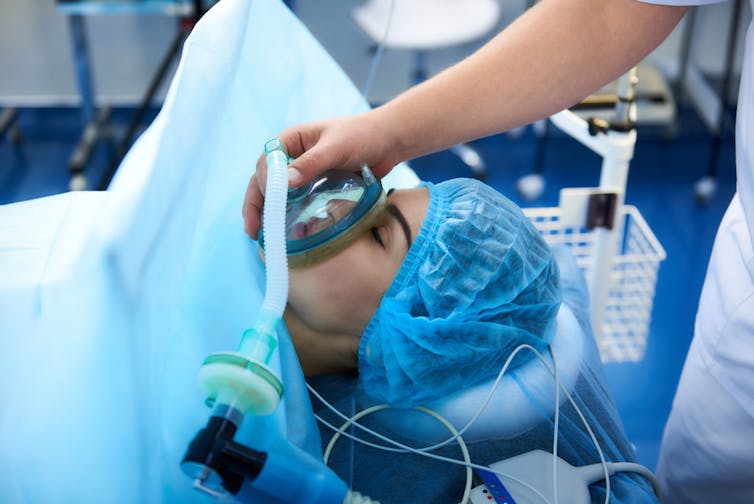
The most common side effects of pholcodine are dizziness, nausea and sedation. But the Therapeutic Goods Administration (TGA) has recalled products containing it, as pholcodine can trigger anaphylaxis around the time of surgery.
The issue arises when pholcodine medicines are combined with types of muscle relaxants given during surgery known as neuromuscular blocking agents.
This type of anaphylaxis can occur in people who have had a muscle relaxant before and been previously fine, or in people who receive a muscle relaxant drug for the first time.
Being obese also places people at higher risk of this type of anaphylaxis.
Shutterstock
Read more:
Cough syrup can harm children: experts warn of contamination risks
We’ve known about the risk for years
We have actually known about the risks of pholcodine and muscle relaxants for some time, including in Scandinavian studies in 2005.
In fact, it was because of these studies that pholcodine was withdrawn from the market in Norway in 2007.
The European Medicines Agency recommended the withdrawal of pholcodine in Europe in December 2022.
In Australia, the PatientSafe Network has been calling for its ban since at least 2017.
Over the past 12 months, there were nine reported cases of serious adverse effects to pholcodine reported to the TGA, including three deaths. The most recent case was in January this year. In three of the nine, pholcodine was the only suspected medicine involved.

Author provided
Why now?
The TGA’s decision may come from a recent (but not yet peer reviewed) French study. This found that when patients had taken pholcodine at any time in the 12 months before surgery that used a muscle relaxant, they were at much higher risk of anaphylaxis.
The French research is consistent with an earlier Western Australian study which found analphylaxis is 14 times more likely to occur when the two types of drugs are combined.
This is because pholcodine can linger in the body for long periods. After you swallow the medicine, the drug reaches its highest concentration in the blood stream one to two hours later. But both the drug and its metabolites can still be detected in the body up to seven weeks later.
I have some at home. What now?
As pholcodine can linger in the body, the TGA has warned that if you have taken a medicine containing pholcodine in the past 12 months you need to tell your doctor before you have surgery.
If you are taking one of these medicines you should stop immediately, even if you don’t think you’re going to have a medical procedure soon.
Take it to your local pharmacy for disposal. At that time, the pharmacist will be able to recommend a different medicine for your cough.
Read more:
Health Check: do cough medicines work?
![]()
Associate Professor Wheate in the past has received funding from the ACT Cancer Council, Tenovus Scotland, Medical Research Scotland, Scottish Crucible, and the Scottish Universities Life Sciences Alliance. He is a Fellow of the Royal Australian Chemical Institute, a member of the Australasian Pharmaceutical Science Association, and a member of the Australian Institute of Company Directors. Nial is the chief scientific officer of Vairea Skincare LLC, a director of SetDose Pty Ltd a medical device company, and a Standards Australia panel member for sunscreen agents.
Associate Professor Tina Hinton has previously received funding from the Schizophrenia Research Institute (formerly Neuroscience Institute of Schizophrenia and Allied Disorders). She is currently a Board member of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists.
– ref. Why cough medicines containing pholcodine can be deadly even if you took them months before surgery – https://theconversation.com/why-cough-medicines-containing-pholcodine-can-be-deadly-even-if-you-took-them-months-before-surgery-200895