Source: The Conversation (Au and NZ) – By Nicholas Wood, Associate Professor, Discipline of Childhood and Adolescent Health, University of Sydney
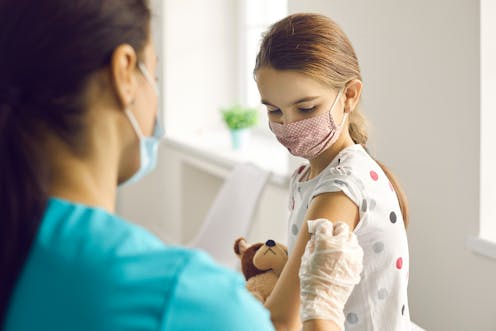
Australian children aged six to 11 years old will be able to get the Moderna COVID vaccine at pharmacies, GP clinics and vaccination hubs from tomorrow.
This follows the Therapeutic Goods Administration’s provisional approval and today’s ATAGI (Australian Technical Advisory Group on Immunisation) recommendation for the Moderna vaccine, called Spikevax, to be made available to this age group.
Australia is one of the first countries to approve the use of Moderna vaccine for children under 12 years old.
Read more:
What is the Moderna COVID vaccine? Does it work, and is it safe?
Until now, the Pfizer vaccine was the only option available for five to 11 year olds.
Moderna eligibility starts at six, so the Pfizer vaccine is still the only COVID vaccine option for kids aged five.
No COVID vaccines are currently available for under-fives and with trials still under way, they’re likely some months away.
What’s the dose and interval?
The Moderna vaccine is given at a lower dosage of 0.25ml (50 micrograms) for children under 12 years old.
(Adults are given 0.5ml for their first and second doses, and 0.25ml for a booster.)
Two doses are recommended for five to 11 year olds, with an eight-week interval.
This can be shortened to four weeks for children at risk of severe COVID and in special circumstances such as travelling overseas.

Shutterstock
How effective is it?
The Moderna vaccine was trialled in a clinical trial called KidCOVE. This trial involved 4,753 children aged six to 11 years and was designed to look at safety and immune responses.
The trial results have not been formally published and only appear in a press release. However, a full clinical dossier will have been submitted to the TGA for its consideration in granting a provisional approval.
The KidCOVE trial found children developed an equivalent level of antibodies to that seen in young adults aged 18 to 25 years. Nearly all participants made antibodies (99.3%) after vaccination.
These results (as detailed in the press release) demonstrate a strong immune response in this group of children one month after the second dose.
Is it safe?
The KidCOVE trial also monitored safety. The Spikevax Moderna vaccine was generally well tolerated, with side effects consistent with those in adolescents and adults.
The most common adverse events were fatigue, headache, fever, and injection site pain.
As often occurs in clinical trials, children in the KidCOVE trial will be followed for 12 months after their second injection to assess long-term protection and safety.
Should my child get Pfizer or Moderna?
There is no direct head-to-head comparison available to tell us which vaccine is more effective in the real-world setting in children age six to 11 years.
Both vaccines were shown to be efficacious, but the overall number of cases available for evaluation in both trials was small.
Side effects reported after the Moderna vaccine were mild to moderate and quickly resolved, as did those those reported in the Pfizer trial of five to 11 year olds.
Read more:
Safety, side effects, allergies and doses. The COVID-19 Pfizer vaccine for 5-11 year olds explained
But while there is no direct comparison of side effects in a single trial, the available evidence suggests mild to moderate side effects are more commonly reported after Moderna. This includes pain at the injection site, swelling and tenderness of lymph nodes in the armpit, fever, headache and nausea
This may be due to the difference in the amount of antigen content (the substance that produces the immune response) between vaccines. Pfizer contains 10 micrograms, while Moderna contains 50 micrograms.
What about rare complications?
Moderna didn’t report any cases of myocarditis or pericarditis (inflammation of the heart muscle or the tissue surrounding the heart) in its clinical trial of 4,700 children. However it’s important to note the sample size of the trial was not large enough to rule out any risk.
Australia’s vaccine monitoring body, AusVaxSafety, has closely monitored the safety of the Pfizer vaccine in children aged 5 to 11 years. It found side effects have generally been mild, with pain, swelling, and redness at the vaccination site the most common.
AusVaxsafety and our other surveillance systems will also monitor Moderna vaccine safety in children as it’s rolled out in the coming months, and will closely watch for serious adverse events, including myocarditis and pericarditis.
Just under half of Australian primary school-age children have received their first COVID vaccine dose, after becoming eligible on January 10. Parents of children now have another vaccine to consider as we look to protect our community from COVID. Hopefully adding another vaccine choice helps to boost these rates.
Read more:
Is your child frightened of needles? Here’s how to prepare them for their COVID vaccine
![]()
Nicholas Wood received funding from the NHMRC for a Career Development Fellowship from 2018 to 2021. He holds a Churchill Fellowship awarded in 2019.
– ref. The Moderna vaccine is now available for 6 to 11 year olds. Here’s what parents need to know – https://theconversation.com/the-moderna-vaccine-is-now-available-for-6-to-11-year-olds-heres-what-parents-need-to-know-177544