Source: The Conversation (Au and NZ) – By Nathan Bartlett, Professor, School of Biomedical Sciences and Pharmacy, University of Newcastle
We’re in the midst of a bad cold and flu season in Australia. Along with the usual viral suspects, such as influenza, RSV, and rhinoviruses (which cause the common cold), bacterial pathogens are also causing significant rates of illness, particularly in children. These include Bordatella pertussis (whooping cough) and Mycoplasma pneumoniae.
Meanwhile, SARS-CoV-2 (the virus that causes COVID) is responsible for recurring waves of infection as it continues to evolve and mutate into new variants which keep it a step ahead of our immunity.
The latest variant is nicknamed “FLuQE”, and is reportedly gaining traction in Australia and other countries. So what is there to know about FLuQE?
From FLiRT to FLuQE
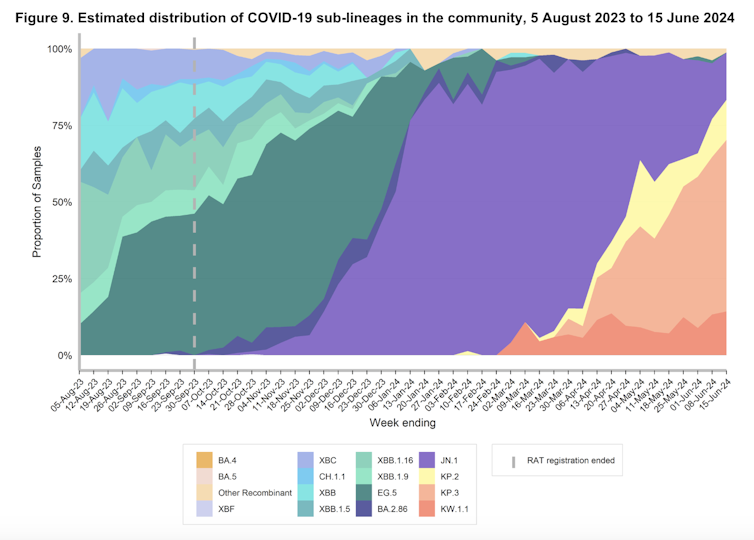
In recent months, you may have heard of the “FLiRT” subvariants. These are decedents of the Omicron variant JN.1, including KP.1.1, KP.2 and JN.1.7.
KP.2, in particular, significantly contributed to COVID infections in Australia and elsewhere around May.
The name FLiRT refers to the amino acid substitutions in the spike protein (F456L, V1104L and R346T). Amino acids are the molecular building blocks of proteins, and the spike protein is the protein on the surface of SARS-CoV-2 which allows it to attach to our cells. These changes in the spike protein arise from mutation – random changes in the genetic code of the virus.
SARS-CoV-2’s goal is to select mutations that produce a spike protein that binds strongly to our cells’ receptors to support efficient infection (sometimes called viral fitness) while avoiding neutralising antibodies in our immune system (immune pressure).
The FLiRT mutations seem to reduce the ability of neutralising antibodies to bind to the spike protein, potentially enabling the virus to better evade our immunity.
But at the same time, it appears the immune pressure which has selected for these mutations may have affected the ability of the virus to bind to our cells.
These findings are yet to be peer-reviewed (independently verified by other researchers). However, they suggest the FLiRT variants may have traded in some ability to infect our cells for a spike protein that’s more resistant to our immune system.

Anna Shvets/Pexels
According to experts in Australia and internationally, what appears to have occurred with FLuQE is an additional mutation has restored fitness that may have been lost with the FLiRT mutations.
FLuQE (KP.3) is a direct descendant of FLiRT, meaning it has inherited the same mutations as the FLiRT variants. But it has an additional amino acid change in the spike protein, Q493E (giving FLuQE its name).
This means the amino acid glutamine at position 493 has changed to glutamic acid (the spike protein is 1,273 amino acids long). Glutamine is a neutral amino acid, whereas glutamic acid has a negative charge, which changes the properties of the spike protein. This could improve the ability of the virus to infect our cells.
It’s still early days for FLuQE and we don’t have peer-reviewed research on this yet. But it appears we now have (another) immune evasive virus that is also well adapted to infecting our cells. It’s no surprise, then, that FLuQE seems to be becoming dominant in many countries.
NSW Health
What next?
We would expect with widespread transmission of and infection with FLiRT and FLuQE variants, population immunity to these variants will mature, and in time, their dominance will be supplanted by the next immune-evasive variant.
The tug of war between our immune system and SARS-CoV-2 evolution continues. The issue we are dealing with now is vaccines don’t sufficiently protect from infection or suppress virus transmission. While they’re very good at protecting against severe disease, the virus still infects lots of people.
As well as the burden on people and health care, lots of infections means more opportunities for the virus to evolve. The more “rolls of the dice” the virus has to find a mutation that helps it evade our immune system and infect our cells, the more likely it is to do so.
Next-generation vaccines and therapies really need to boost immunity in the upper respiratory tract (nose and throat) to reduce infection and transmission. This is where infection initiates. A human challenge study, where volunteers are experimentally exposed to SARS-CoV-2, showed people who didn’t become infected had a robust anti-viral immune response in their upper respiratory tract.
To this end, there are immune-stimulating nasal sprays and nasal vaccines in clinical development. The hope is this approach will slow down the evolution of SARS-CoV-2 and the emergence of new subvariants that continue to drive waves of infection and disease.
Fortunately, so far these mutations have not generated a virus that is obviously more pathogenic (causes worse disease), but there are no guarantees this won’t happen in the future.
![]()
Nathan Bartlett does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
– ref. From FLiRT to FLuQE: what to know about the latest COVID variants on the rise – https://theconversation.com/from-flirt-to-fluqe-what-to-know-about-the-latest-covid-variants-on-the-rise-234073