Source: The Conversation (Au and NZ) – By Karen Canfell, Adjunct professor, cancer epidemiology, University of Sydney
Cervical screening in Australia has changed over the past seven years. The test has changed, and women (and people with a cervix) now have much more choice and control. Here’s why – and what you can expect if you’re aged 25 to 74 and are due for a test.
When and why did the test change?
In 2017, Australia became one of the first two countries to transition from Pap smears to tests for the presence of the human papillomavirus (HPV).
HPV causes virtually all cervical cancers, so testing for the presence of this virus is a very good indicator of a person’s current and future risk of the disease.
This contrasts with the older Pap smear technology, which involved inspection of cells every two years for the changes resulting from HPV infection.
The change to screening was supported by a very large body of international and Australian data showing primary testing for HPV is more accurate than Pap smears.
Women and people with a cervix who do not have HPV detected on their test are at a very low risk of developing cervical cancer over the next five years, or even longer. This was the basis for lengthening the screening interval when HPV screening was introduced.
Australia now recommends five-yearly HPV screening, starting at age 25 up to the age of 74 for eligible people, whether or not they have been vaccinated against HPV. Many other countries are following suit to transition to HPV screening.
All established screening tests – which are conducted in people without any symptoms – are associated with health benefits but also with some harms. These can include the psychological and clinical consequences of receiving a “positive” screening result, which needs to be investigated further.
New Africa/Shutterstock
However, recent World Health Organization (WHO) reviews of the evidence have found:
- HPV is a more effective screening test than Pap smears or any other method
- it substantially reduces incidence and death rates from cervical cancer
- it is the method of cervical screening that has the best balance of benefits to harms.
As a consequence, the WHO now unequivocally recommends HPV screening as the best-practice method.
Now you can collect your own sample
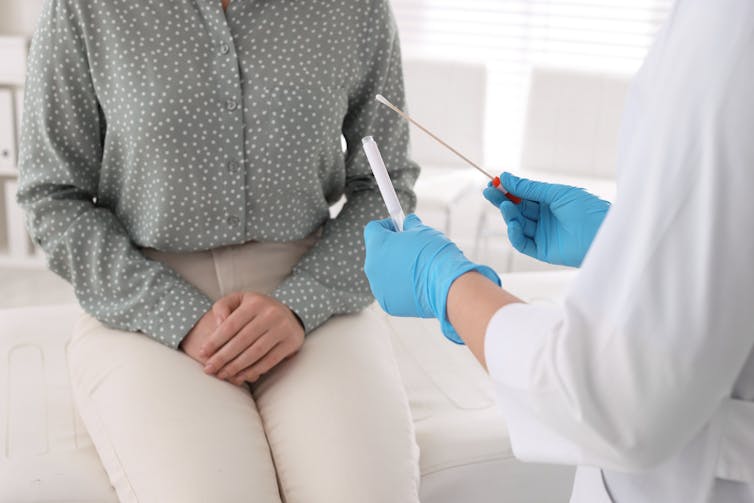
One of the major benefits of switching to HPV screening is it opened the door for a person being able to collect their own sample (which was impossible with the Pap smear). If HPV is present, it can be detected in the vagina rather than having to directly sample the cervix.
In 2022, Australia became one of the first countries in the world to introduce a universal option to choose self-collection within a major national-level screening program. This means people eligible for screening, under the guidance of a primary care practitioner, can now choose to collect their own vaginal sample, in privacy, using a simple swab.
By the end of 2023, 27% of people were choosing to take the test this way, but this is on an upward trajectory and is likely to increase further, with an awareness campaign due to start next month.
So what happens when I have a test?
You’ll receive an invitation from the National Cancer Screening Register to attend your first screen when you turn 25. If you’re older, you’ll receive reminders when you are due for your next test. You will be invited to visit your GP or community health service for the test.
You should be asked whether you would prefer to have a clinician collect the test or whether you would prefer to take the sample yourself.
There’s no right or wrong way. The accuracy of testing has been shown to be equivalent for clinician or self-collected sampling. This is a matter of choice.
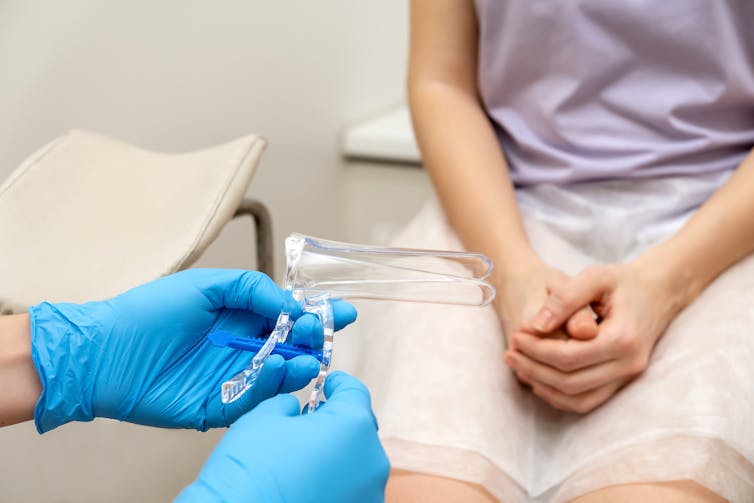
If the clinician does the test, they will undertake a pelvic examination with a speculum inserted into the vagina. This enables the doctor or nurse to view the cervix and take a sample.
Tatiana Buzmakova/Shutterstock
If you are interested in the self-collection option, check whether the practice is offering it when making an appointment.
If you opt for self-collection, you’ll be able to do so in private. You’ll be given a swab (which looks like a COVID test swab with a longer stem), and you’ll be given instructions about how to insert and rotate the swab in the vagina to take the sample. It takes only a few minutes.
What does it mean if my test detects HPV?
If your test detects HPV, this means you have an HPV infection. These are very common and by itself doesn’t mean you have cancer, or even pre-cancer (which involves changes to cervical cells that make them more likely to develop into cancer over time).
It does mean, however, that you are at higher risk of having a pre-cancer, or developing one in future, and that you will benefit from further follow-up or diagnostic testing. Your doctor or nurse will guide you on the next steps in line with national guidelines.
If you require a diagnostic examination, this will involve a procedure called colposcopy, where the cervix is closely examined by a gynaecologist or other specially trained healthcare practitioner, and a small sample may be taken for detailed examination of the cells.
If you have a pre-cancer, you can be treated simply and quickly, usually without needing to be admitted to hospital. Treatment involves ablating or removing a small area of the cervix. This treatment will drastically reduce your risk of ever developing cervical cancer.
What does this mean for cervical cancer rates?
Cervical screening for HPV is a very effective method of preventing cervical cancer. Because of Australia’s HPV screening, combined with HPV vaccination in younger people, Australia is expected to achieve such low rates of cervical cancer by 2035 that it will be considered eliminated.
Last year, the government launched a national strategy for cervical cancer elimination which provides key recommendations for eliminating cervical cancer, and for doing so equitably in all groups of women and people with a cervix.
One of the best things you can do to protect yourself is to have your cervical screening test when you become eligible, whether or not you have been vaccinated against HPV.
Marion Saville, a pathologist and Executive Director at the Australian Centre for the Prevention of Cervical Cancer, co-authored this article.
![]()
Karen Canfell receives funding from a range of government and non-government sources. She is co-principal investigator of an investigator-initiated trial of cervical screening, Compass, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity; the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, and operational support from the Australian Government. She is also co-principal investigator on a major investigator-initiated implementation program Elimination of Cervical Cancer in the Western Pacific (ECCWP) and an extension of this work, the Elimination Partnership in the Indo-Pacific for Cervical Cancer (EPICC). This receives support from the Australian Government’s Department of Foreign Affairs and Trade, the Minderoo Foundation and equipment donations from Cepheid.
Deborah Bateson Deborah Bateson is a co-investigator on the implementation program Elimination of Cervical Cancer in the Western Pacific, which has received support from the Minderoo Foundation and equipment donations from Cepheid Inc.
Megan Smith receives funding for research from the Commonwealth Department of Health, and has previously received support from the National Health and Medical Research Council, Cancer Institute NSW and the Australian Centre for the Prevention of Cervical Cancer.
– ref. I’m due for a cervical cancer screening. What can I expect? Can I do it myself? And what happened to Pap smears? – https://theconversation.com/im-due-for-a-cervical-cancer-screening-what-can-i-expect-can-i-do-it-myself-and-what-happened-to-pap-smears-229495




