Source: The Conversation (Au and NZ) – By Jenny Graves, Distinguished Professor of Genetics and Vice Chancellor’s Fellow, La Trobe University
In most mammals, including humans, females have two copies of the X chromosome while males have only one (accompanied by a Y). So you might think females get a double dose of proteins encoded by the 850-odd genes on the X chromosome, while males get only a single dose.
How can this be fair? Indeed, this gene dosage difference between men and women would cause genetic mayhem if it were not for a system that balances out the dosage difference.
In humans, mice and other mammals, one of the two X chromosomes in females is “silenced”. Silenced genes on this chromosome don’t produce any of the RNA that contains instructions for making proteins. Biologists long thought that the complexity of the molecular failsafe system that achieves this silencing meant X chromosome inactivation was essential for life.
But it turns out things aren’t that simple. Some animals, such as platypus and birds, seem to function just fine with genes on both X chromosomes producing RNA. However, in a new paper, we report that these animals still balance out the dose of X chromosome proteins – they just have a different way of doing it.
X chromosome inactivation occurs in mice, humans – and kangaroos
In 1961, English geneticist Mary Lyon first suggested that one X chromosome in XX females is genetically switched off early in development and stays that way throughout life. The inactive X was quite easy to spot under the microscope, due to its “scrunched up” shape and other differences.
Inactive X chromosomes were eventually found in every species of placental mammal – and even in some non-placental mammals such as kangaroos.
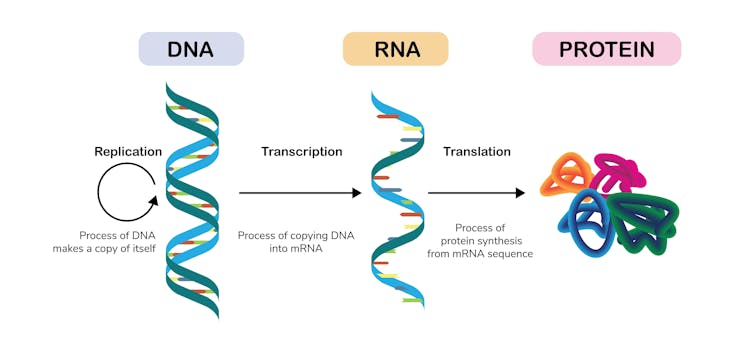
Normally, inside a cell, an enzyme called a polymerase “reads” sections of the DNA – genes – and transcribes them into RNA molecules. These RNA molecules then act as instructions for molecular machines called ribosomes to build different protein molecules out of amino acids. The proteins are what do most of the work in the cell.
BigBearCamera / Shutterstock
In 1986, one of us (Graves), produced the first evidence that inactivation of the extra X chromosome was due to failure of the genes on the inactive X to be copied into RNA. (At the time, referees for a high profile journal turned this paper down as too obvious, writing “What else could it be?”)
We now know several different molecular mechanisms conspire to prevent transcription of genes on the inactive X. These include adding small groups of atoms to parts of the DNA, various modifications of proteins that bind to the DNA, and gluing the inactive X to the membrane that surrounds the nucleus of the cell. All of this is coordinated by a giant RNA molecule that doesn’t code for a protein.
This failsafe mechanism, with its many backups, bolstered the idea that X inactivation was essential for life.
… but not in platypus or birds
In 2008, there was confusion when a research group including two of the authors (Graves and Waters) discovered that the system didn’t seem to be working in platypus. Some X chromosome genes were fully compensated (meaning females and males had the same amount of RNA for those genes), some were not compensated at all, and most were partially compensated – so females had more RNA copied from those genes than males did, but not twice as much.
This was particularly surprising because the platypus has five X chromosomes that together account for nearly 10% of the animal’s genes. So the need for dosage compensation would seem to be acute.
Other research showed birds seemed to have a similar imbalance of RNA transcribed from genes on sex chromosomes.
How could birds, let alone platypus, get away with such blatant sex differences? Maybe the unequal gene dosage in males and females was not so important after all?
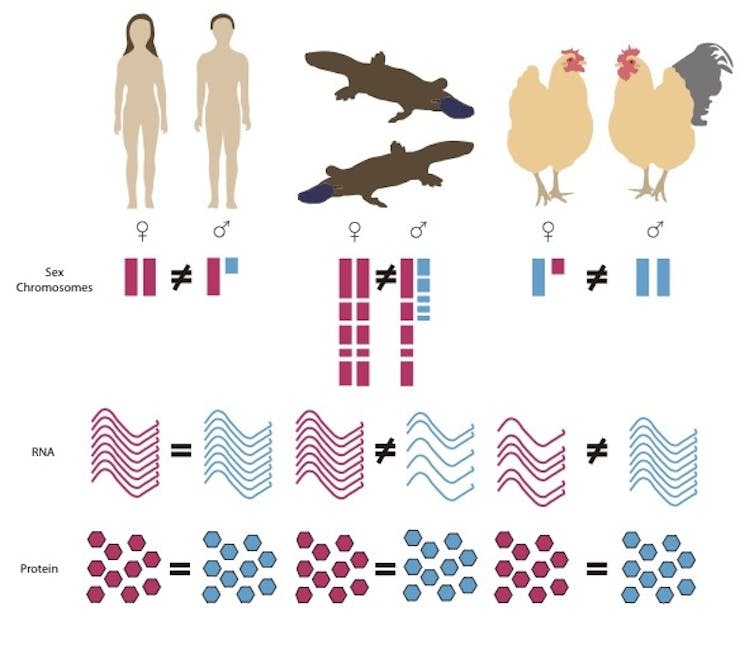
Platypus male and females show dosage equality at the protein level
Well, not so fast. In a new paper we show that compensation for the unequal dosage of sex chromosomes is essential – but it can happen at the level of making proteins, as well as at the level of stopping RNA being transcribed from the X chromosome.
When we measured RNA transcribed from X chromosome genes in platypuses, we confirmed that the amounts were unbalanced in females and males, and different genes were compensated to different extents.
But when we measured proteins made by these RNA molecules, we found the amount was exactly equal in males and females. So while there are different numbers of RNA transcripts in males and females, the protein products of RNA translation are somehow adjusted to be equal.
Nicholas Lister
We got the same result in birds. Genes on chicken sex chromosomes are transcribed very unequally into RNA molecules in males and females, but their protein products are exactly balanced.
So partial compensation at the level of RNA transcription is balanced by some sort of “post-transcriptional control” that occurs after RNA synthesis to cancel out the difference.
It will be fascinating to discover how this works. It might be that RNAs are translated differently into protein, or that RNA transcripts or proteins have different stability in males and females. Because different genes are partially compensated to different extents, post-transcriptional control needs to be finely tuned for each gene.
These results suggest dosage compensation of sex chromosomes between males and females is a necessity, not just in birds and monotremes but all vertebrate species.
![]()
Jenny Graves receives funding from the Australian Research Council.
Paul Waters receives funding from Australian Research Council.
Nicholas Lister does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
– ref. What’s the secret of genetic equality between the sexes? New platypus chromosome research may hold the key – https://theconversation.com/whats-the-secret-of-genetic-equality-between-the-sexes-new-platypus-chromosome-research-may-hold-the-key-235214





